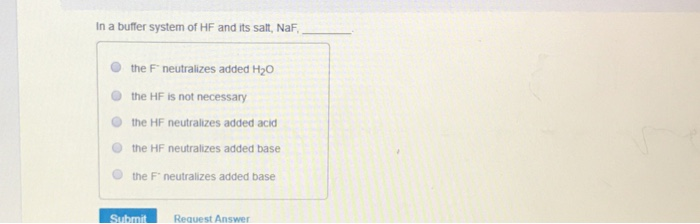
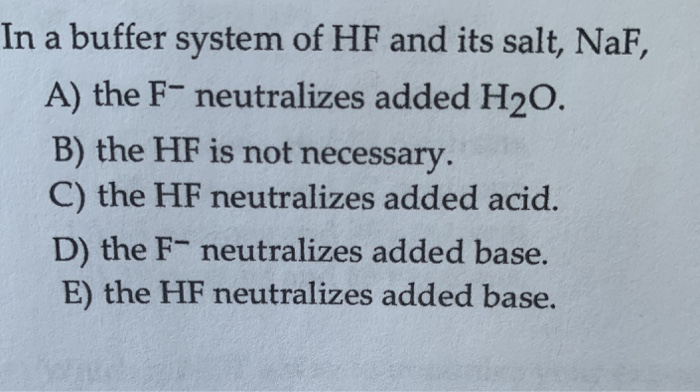
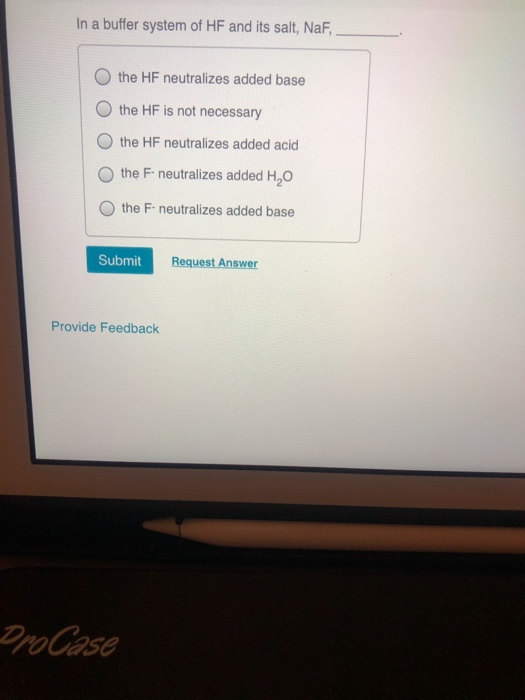
In A Buffer System Of Hf And Its Salt Naf
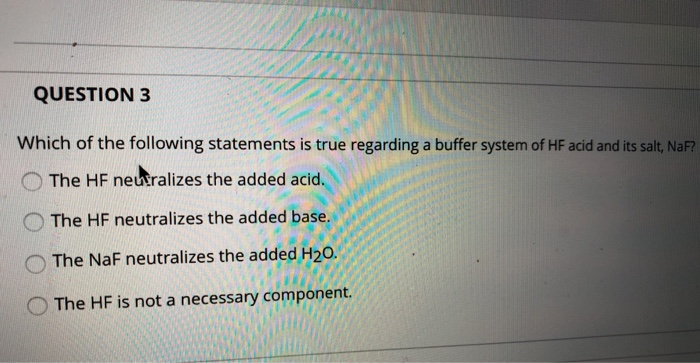
In a buffer system of hf and its salt naf. C the HF is not necessary. Academiaedu is a platform for academics to share research papers. 100 g C14H18N2O5 c.
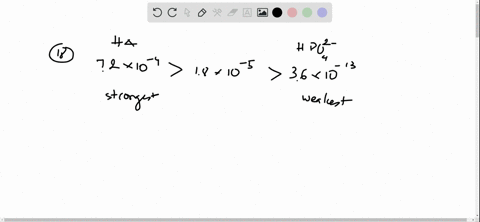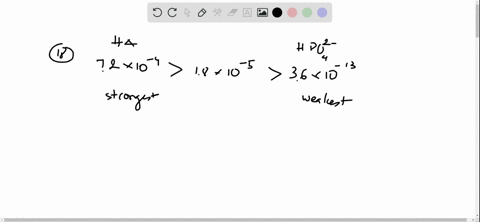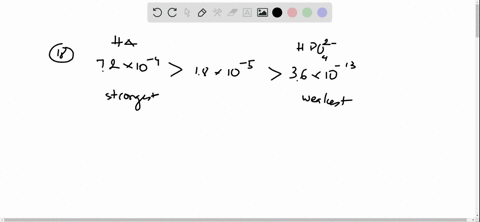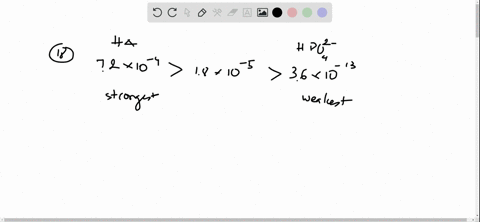
In 10 mM HCl the indicator is in its base form beaker b. Free essays homework help flashcards research papers book reports term papers history science politics. Ka for hypobromous acid HBrO is 2.
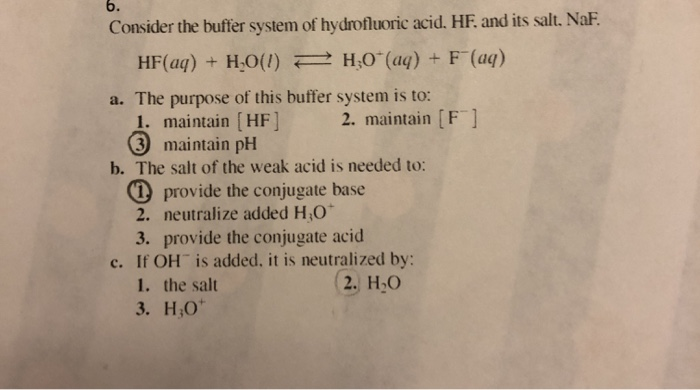
The periodic table is composed of metals semi-metals and nonmetal elements. The gel was incubated in renatured buffer containing 25 mM Tris-HCl pH 75 1 mM DTT 5 mM NaF and 01 mM Na 3 VO 4 at 4 C for 1 h 12 h and 1 h respectively. A buffer contains 0150 moles of HF pKa 315 and 0070 moles of KF in 15 L of solution.
Dissolve 05712 g NaHCO. Transcript 10 mol F2 440 g CO2 40 g H2 146 g SF6 34. 02210 g NaF100 mL b.
In contrast the solubility of salt NaCl in water is only about 36 36 g s. Dissolve salt in filtered deionized water a. E-Book Overview Dan Harriss Quantitative Chemical Analysis continues to be the most widely used textbook for analytical chemistry.
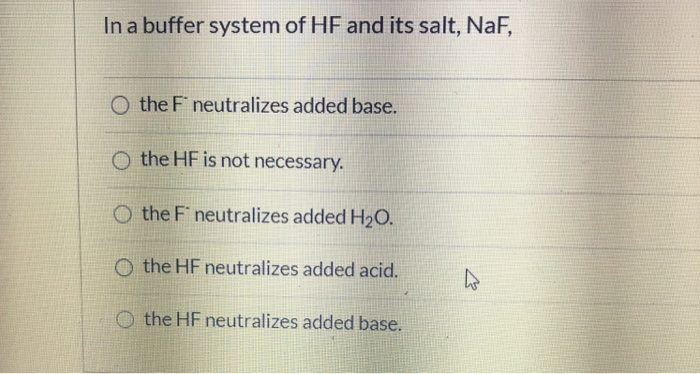
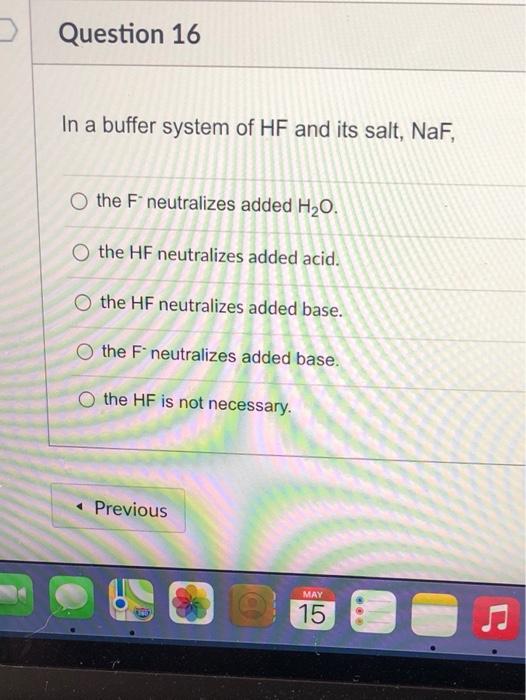
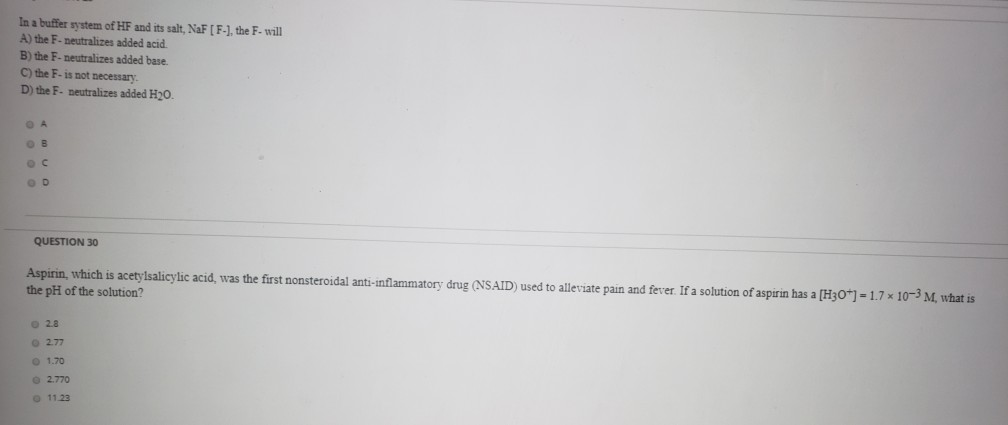
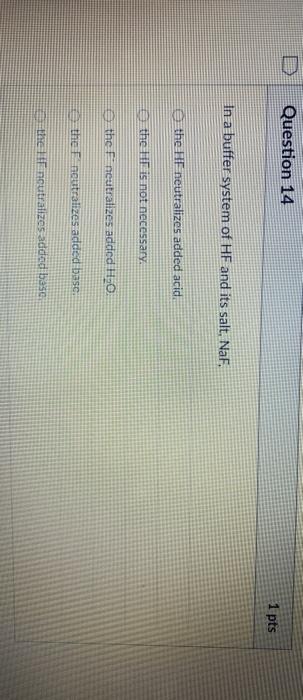
And 07631 g Na. In a buffer system of HF and its salt NaF _____. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry.
In blood this pH range is between 735 and 745. A The buffered solution on the left and the unbuffered solution on the right have the same pH pH 8.
Bicarbonatecarbonate buffer solution 17 mM NaHCO.
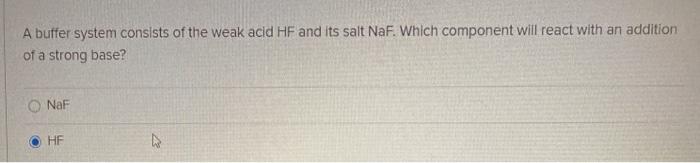
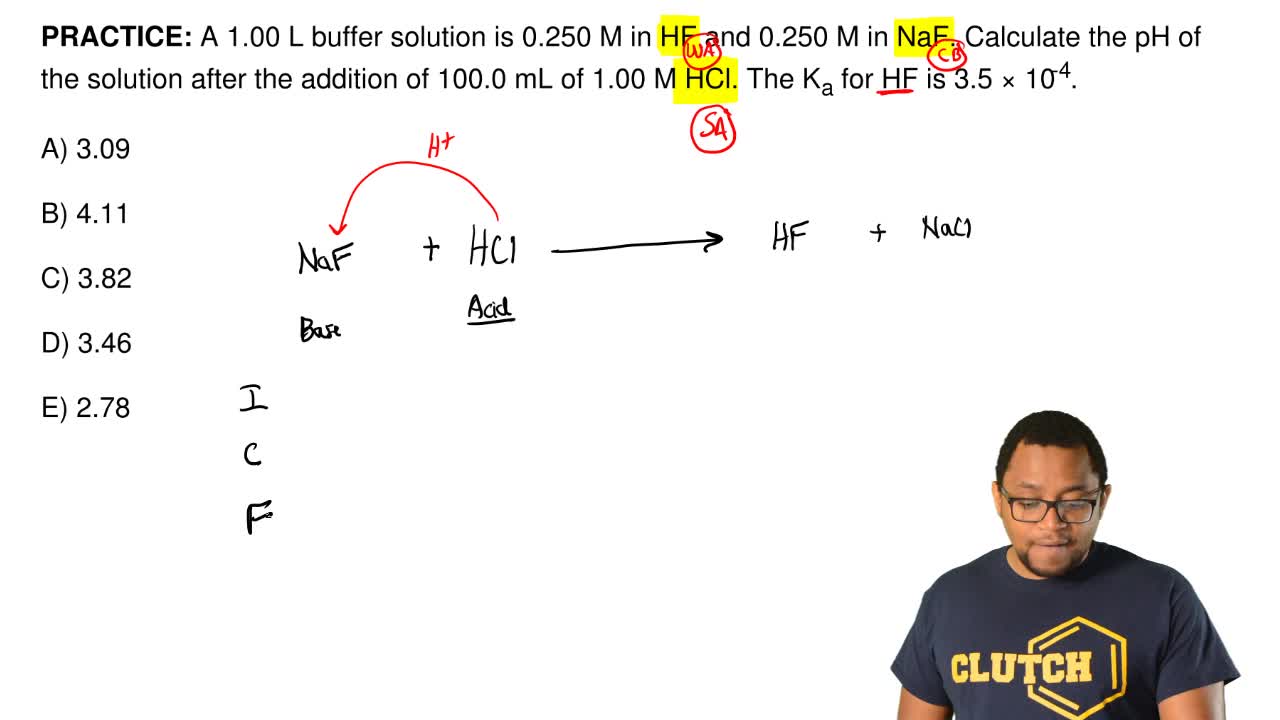
PH 357 Your buffer contains hydrofluoric acid HF weak acid and sodium fluoride NaF the salt of its conjugate base the fluoride anion F-. Calculate the pH of a solution containing 040 mol fluoride anion and 030 mol of hydrogen fluoride HF. The main buffer system in blood is the carbonic acid hydrogen carbonate buffer system. PH 357 Your buffer contains hydrofluoric acid HF weak acid and sodium fluoride NaF the salt of its conjugate base the fluoride anion F-. Ka for hypobromous acid HBrO is 2. In 10 mM HCl the indicator is in its base form beaker b. In 4 L filtered deionized water 5. Calibration stock solutions 1 mgmL as the anion. Transcript 10 mol F2 440 g CO2 40 g H2 146 g SF6 34.
Oh- According to LeChâteliers principle predict whether adding H2O causes the system to shift in the direction of reactants products or no change. In a buffer system of HF and its salt NaF _____. Academiaedu is a platform for academics to share research papers. 2 10-10 M. In 10 mM HCl the indicator is in its base form beaker b. For co-IP experiments the 35SHF-COP1 35SHF-ICE1 Ding et al 2015 SuperCRY2-mCherry and SuperHY5-Myc plasmids were purified and transformed into Arabidopsis mesophyll protoplasts. Molar mass of naf.














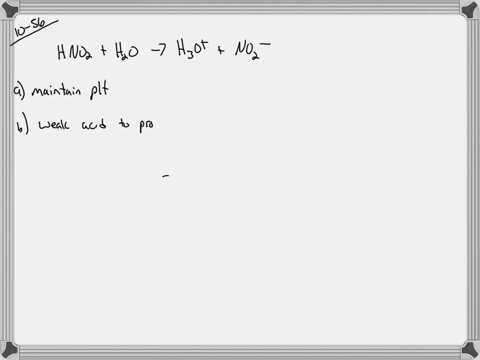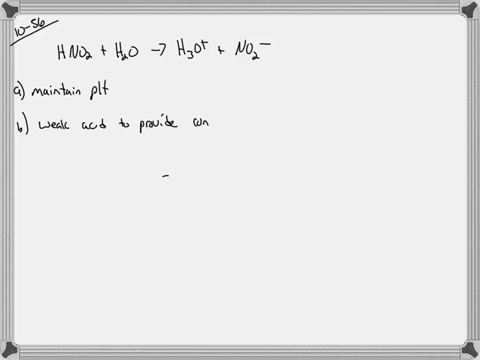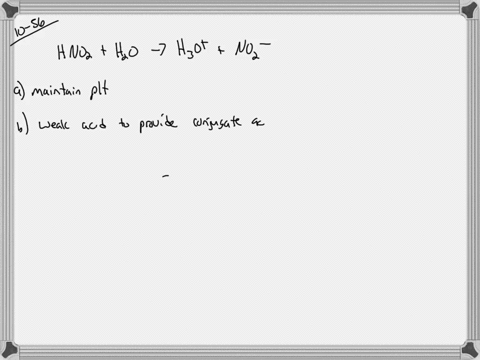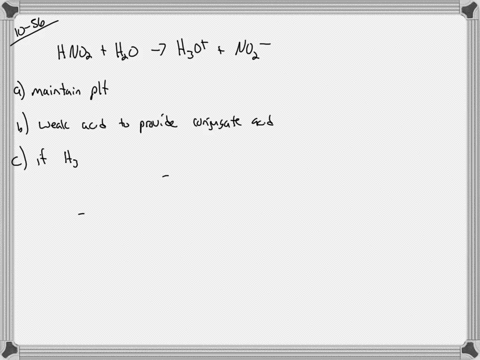





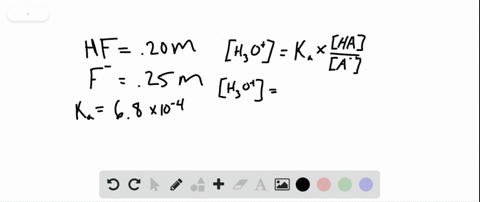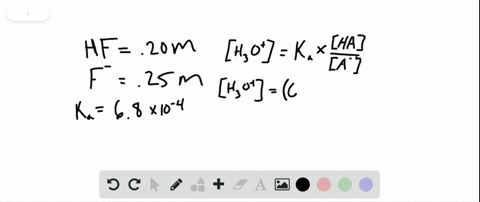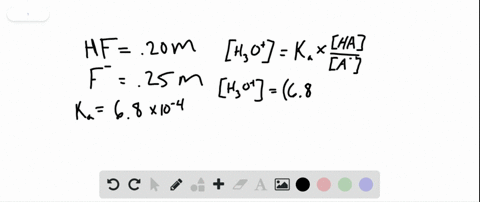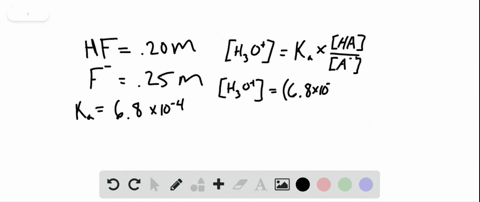












Post a Comment for "In A Buffer System Of Hf And Its Salt Naf"